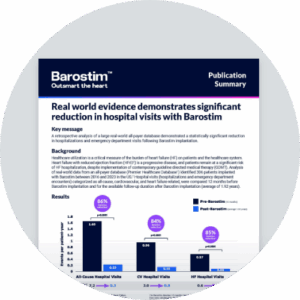
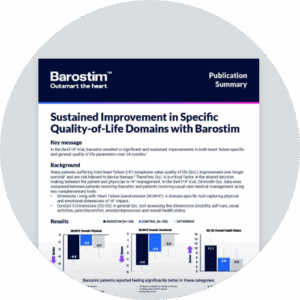
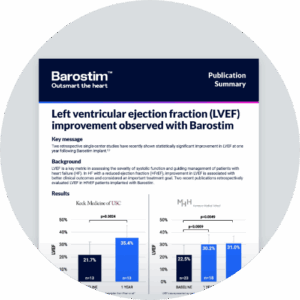
Clinical results
BeAT-HF trial
Sustained symptomatic improvements
Barostim plus GDMT provides significant and meaningful improvements for heart failure patients beyond GDMT alone
12 months
Nominal p-value < 0.001
Exercise capacity
(6MHW)1
Nominal p-value < 0.001
Quality of life
(MLWHF)1
Nominal p-value < 0.001
Functional status
(NYHA class)1
Freedom from all-cause death,
LVAD, and transplant1
HR 0.662 (95% CI 0.435, 1.007)
nominal p=0.054
Patients in the Barostim arm had a 34% reduction in all-cause death or the use of LVAD or heart transplant at > 4 years follow-up
Safe procedure1
Freedom from major adverse neurological or cardiovascular system or procedure-related event rate in the Barostim arm
1. Zile MR, et al. Eur J Heart Fail. 2024 Apr 12; 2. Gremeaux V, et al. Arch Phys Med Rehabil. 2011;92(4):611-619.; 3. Rector TS, et al. J Card Fail. 1995;1(3):201-216;